-40%
Zinc Wire 0.0079-0 3/8in 99.9% for Electrolysis Plating Craft Anode Jewellery
$ 1609.13
- Description
- Size Guide
Description
Zinc wire 0.2-10mm 99.9% for electroplating craft wire anode jewelry wireZinc wire 0.2-10mm 99.9% for electroplating craft wire anode jewelry wire
Do you need a different steel quality or dimension? Please write to us. Our sales team makes an offer from stock program or delivery program :)
Length tolerance +/-2mm
TOP quality at fair prices
High-quality products made to measure and cut to size
Competent advice & service
Technical specifications:
Brand:
Evek
Manufacturer number:
Not applicable
Type:
wire
Material:
zinc
Operation area:
as protection against corrosion of iron and steel products (buckets, wires, pipes); in batteries (alkaline manganese batteries, silver oxide zinc batteries); in construction (zinc sheet for roofing and facade cladding); in organic chemistry as a reducing agent; production of hydrogen.
Norm classifications:
Name, symbol, atomic number:
Assay:
99.9%
US:
S30400
Further information:
melting point:
692.68K (419.53°C)
Boiling point:
1180K (907°C)
Density:
7.14 g/cc
thermal conductivity:
120 W/m K
electr. Resistance:
0.055Ωmm²/m
Description:
Production time - 5-7 weeks.
Preparation for shipment - 2-3 working days.
Look at the FAQ please.
Zinc is a brittle transition metal from the zinc group. This is a ductile heavy metal that can be easily deformed at 120 °C to 150 °C and can therefore be rolled into sheets and wires. Zinc metal is stable in air due to passivation. At a temperature of 220 °C, it can be easily processed into powder. In powder form, zinc is pyrophoric, meaning it can spontaneously ignite in air at room temperature. Zinc is soluble in acids, forming salts and hydrogen. It dissolves in alkalis with the formation of zincates. However, high-purity zinc (99.999%) is acid-resistant.
Zinc metal is mainly used for galvanizing iron and steel products to protect them from corrosion. Zinc can also be easily alloyed with other metals. In this way, brass is obtained from copper and zinc, which is in demand in the sanitary sector and in the jewelry industry. Zinc is also used in non-rechargeable batteries (e.g. in alkaline manganese batteries).
Zinc is used:
as protection against corrosion of iron and steel products (buckets, wires, pipes);
in batteries (alkaline manganese batteries, silver oxide zinc batteries);
in construction (zinc sheet for roofing and facade cladding);
in organic chemistry as a reducing agent;
production of hydrogen.
Zinc metal is characterized by:
high ductility;
resistance to air;
pyrophoric properties in powder form;
acid resistance in highly pure form;
good deformability.
documentation
Data sheet (German)
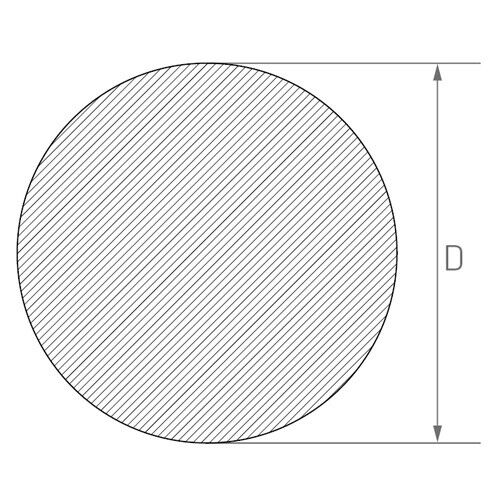
d — outer diameter
Listing and template services provided by inkFrog
Zinc is a brittle transition metal from the zinc group. This is a ductile heavy metal that can be easily deformed at 120 °C to 150 °C and can therefore be rolled into sheets and wires. Zinc metal is stable in air due to passivation. At a temperature of 220 °C, it can be easily processed into powder. In powder form, zinc is pyrophoric, meaning it can spontaneously ignite in air at room temperature. Zinc is soluble in acids, forming salts and hydrogen. It dissolves in alkalis with the formation of zincates. However, high-purity zinc (99.999%) is acid-resistant. Zinc metal is mainly used for galvanizing iron and steel products to protect them from corrosion. Zinc can also be easily alloyed with other metals. In this way, brass is obtained from copper and zinc, which is in demand in the sanita